|
||||||||||
|
|
||||||||||
|
||||||||||
|
|
||||||||||
The use of aluminum in solid rocket propellants dates back to the early 1950s. While working under contract for the US Navy, two young engineers from the Atlantic Research Corporation named Keith Rumbel and Charles Henderson succeeded where other scientists had failed. Contemporary theory of the day had shown that slight increases in specific impulse could be accomplished by adding small amounts of aluminum, less than 5% by weight, to solid propellants. Due to the marginal performance increase predicted by this theory, however, little research had been spent on aluminized propellants.
Undiscouraged, Rumbel and Henderson forged ahead with their own series of experiments to add large amounts of aluminum, 21% by weight, to a castable composite propellant. Their results indicated a dramatic increase in the exit velocity of the solid rocket combustion gases that compared well with the performance of liquid fuels like kerosene and liquid oxygen. Large increases in specific impulse were also measured that promised to significantly increase the range of rockets and ballistic missiles.

At the time of this discovery, the Navy was preparing to place the first generation of strategic missiles aboard submarines. The Navy had wisely planned to use solid rocket propulsion systems aboard ships to avoid the complexity and safety problems inherent with liquid fueled rockets. Unfortunately, preliminary design studies had indicated that these intercontinental ballistic missiles would have to be very large to achieve the range required to reach targets within the Soviet Union. Larger missiles meant larger submarines would be needed to carry them, and the costs of these systems would increase significantly.
The research in aluminized propellants proved to be a vital breakthrough that made the Navy's missile submarines practical. Thanks to this new technology, the Navy was able to develop smaller missiles carrying the same sized warheads over the same range. These smaller missiles could be carried aboard smaller vessels and were far safer to operate and maintain at sea than a liquid fuel system. Rumbel and Henderson's research resulted in the deployment of the Polaris A1, the first ever submarine-launched ballistic missile (SLBM).

The wisdom of using solid rocket missiles aboard submarines is exemplified by the superb safety record of the US Navy ballistic missile submarine fleet. The Soviet Navy, however, has relied upon liquid rocket fuels aboard its missile submarines and suffered a disastrous accident as a result. The nuclear-powered submarine K-219 was on patrol off the American East Coast in 1986 when one of the missile hatch covers sprung a leak. The leak allowed saltwater to react with liquid fuel that had dripped from the missile into the launch tube. The reacting fluids created toxic gases that caused an explosion and started fires aboard the submarine. Four crewmen died while trying to save the vessel, and the spreading toxic gases forced the remainder to evacuate. As the Soviets attempted to tow the submarine back to port, the boat sank in the Atlantic Ocean taking two nuclear reactors and 32 nuclear warheads to the bottom. Had the submarine instead used solid rocket motors rather than liquid fuel, the leaking seawater would not have led to the catastrophic chain of events.
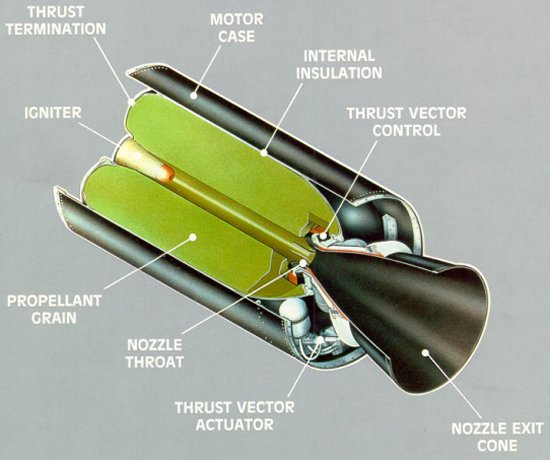
Today, aluminum still remains a main ingredient in many tactical missiles and space-launch platforms. The most common type of aluminum used in solid propellant formulations is powdered spherical aluminum 5 to 60 mm in diameter and usually constitutes 14% to 20% of the propellant by weight. The Space Shuttle's Solid Rocket Boosters, for example, contain over 1.1 million pounds (500,000 kg) of propellant and produce over 3.3 million pounds (14.7 million newtons) of thrust each using a 16% aluminum formulation. A similar formulation will continue to be used in the Shuttle Derived Launch Vehicles that NASA proposes to use as replacements for the Space Shuttle by 2015.
The increase in solid rocket performance that aluminum provides is due to the material's combustion properties. Aluminum burns at over 4,100 K (6920°F), which is two-thirds the temperature at the surface of the Sun. At these temperatures, the burning aluminum releases a large amount of energy that significantly expands the gases within the combustion chamber. As the combustion gases expand within the fixed volume, the pressure rises and forces the exhaust gases to escape through the nozzle at a much higher exit velocity. The higher exit velocity, in turn, raises the specific impulse of the motor.
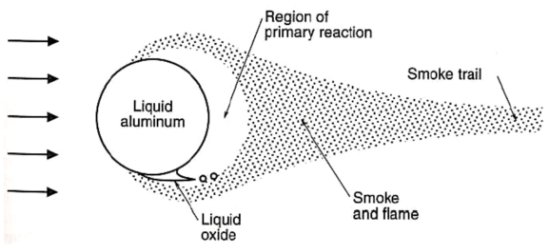
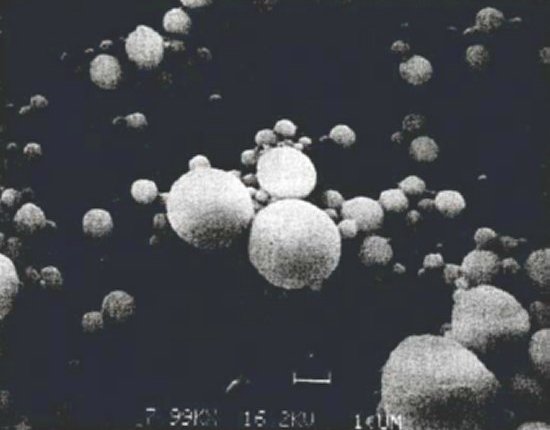
As the solid aluminum is combusted, it oxidizes to form aluminum oxide (Al2O3 or alumina). The exact nature of the oxidation process is still the subject of ongoing investigation, but it is generally believed that most of the aluminum at the burning surface of the propellant is immediately converted to aluminum oxide smoke particles less than 5 mm in diameter. The remaining aluminum coalesces into larger agglomerates that may reach as much as 500 mm in diameter. These agglomerates are pulled off of the burning surface by the surrounding combustion gases and then carried away. The agglomerates remain in a molten state and burn more slowly as they travel downstream through the chamber and out the nozzle.
The presence of these aluminum agglomerates within the chamber volume is beneficial since the molten chunks tend to dampen out combustion instabilities that may occur. Solid rockets often suffer from unsteady acoustical waves that may resonate within the combustion chamber and cause pressure spikes. The advantage of aluminum agglomerates is that they interfere with these traveling waves and create a more steady, even burning of the propellant.
It should be noted that other additives can also be used in solid rocket propellants to produce a similar boost in performance. Beryllium is one such material. Aluminum remains a more attractive option since it is an inexpensive fuel and relatively non-toxic compared to beryllium.
However, the combustion of aluminum presents disadvantages as well. While most of the smaller alumina particles follow the path of the combustion gases through the nozzle, the momentum of the larger particles often causes them to collide with the walls of the motor casing and nozzle. These collisions can erode away the surfaces of these walls reducing the efficiency of the rocket motor. The molten aluminum might also collect in a pool in a submerged nozzle configuration and slosh around within the submergence. Due to the high temperature, these molten pools often react with the composite casing causing premature decomposition of the motor walls. Viscous forces can also pull the pooled molten aluminum into the nozzle throat causing a partial clog of the region. This clog reduces the throat area resulting in a temporary pressure spike within the chamber.
Once the alumina particles pass through the throat, they are further accelerated with the flow of the combustion gases. The larger particles tend to move along the centerline of the nozzle while the smaller particles are pulled away towards the nozzle walls. As the gases continue to expand outward, the temperature within the exhaust flow often drops and the alumina may solidify. The high-speed aluminum oxide droplets can collide with the nozzle walls causing accelerated erosion.
Due to this impingement, solid rocket motors with aluminized propellants often require a nozzle shape that is more conical than the ideal bell nozzle often used in rocketry. Unfortunately, conical nozzles are typically less efficient because of divergent losses. These losses occur when the exiting flow no longer runs tangentially with the nozzle axis. Conical nozzles also tend to be longer, heavier, and more expensive than a bell nozzle.
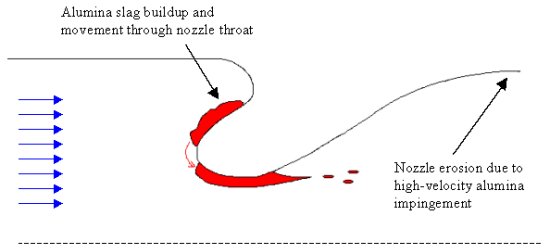
The greatest disadvantage of aluminized propellants, however, results from the two-phase flow loss associated with the agglomerates. When these large droplets form, they are traveling at relatively slow speeds compared to the exhaust gases within the combustion chamber. It takes energy to accelerate these relatively large particles to the speed of the gas flow. The energy needed to change the momentum of the aluminum agglomerates is extracted from the gases as they flow downstream. Moreover, the aluminum particles are not only being accelerated axially, but radially as well, which can rob the exhaust of considerable energy depending on the aluminum content of the propellant. In fact, two-phase flow losses can account for as much as a 5% reduction in rocket performance.
Despite the problems associated with aluminized propellant combustion, the benefits of increased specific impulse
far outweigh the costs. The US government is also currently funding many research projects aimed at reducing the
severity of these issues in order to make aluminized solid rockets even more advantageous. Over the past few
decades, significant advances in computer modeling and measurement techniques have led to greater understanding of
aluminum combustion phenomena. Thanks to this research, the next generation of solid rocket motors will likely be
more efficient and control even the smallest details of the combustion process. These enhancements will provide
even greater rocket performance in the future that improves the capabilities of missiles and permits easier access
to space.
- answer by Matt Walker, 9 October 2005
Related Topics:
Read More Articles:


|
Aircraft | Design | Ask Us | Shop | Search |

|
|
| About Us | Contact Us | Copyright © 1997-2023 | |||
|
|
|||